What Is The Stored Carbohydrate In Animals
3. Carbohydrates, Structures and Types
This chapter provides an introduction and discussion of carbohydrates that are important in the nutrition of food-producing animals.
New Terms
Amylopectin
Amylose
Cellulose
Disaccharide
Fructose
Galactose
Glucose
Glycogen
Heteropolysaccharide
Homopolysaccharide
Monosaccharide
Oligosaccharide
Polysaccharide
Starch
Trisaccharide
Affiliate Objectives
- To present the chemic structure of different types of carbohydrates and their importance in brute nutrition
Carbohydrates
What Are Carbohydrates?
Carbohydrates are the major components of found tissue, making upwards to 60% to xc% of the dry matter (DM). Carbohydrates comprise carbon, hydrogen, and oxygen in the proportion found in water (CHiiO) and are hence hydrates of carbon. Carbohydrates are the basic energy source in animal cells. Dietary carbohydrates obtained from establish-based products serve as a major source of energy for the animal. The chlorophyll in constitute cells traps solar energy and produces carbohydrates using carbon dioxide and water and gives off oxygen, as shown in the following equation:
solar energy + 6 CO2 + 6 H20 → C6H2O + 6 O2.
Carbohydrates are the major dietary source of energy for animals.
In the plant cell, carbohydrates could be present in the cell content as sugar or starch, or they could be associated with the jail cell wall structure (e.g., cellulose). When animals eat constitute materials (e.grand., cereal grains, grass, forage), energy in the feed's carbohydrates is made available through metabolic processes in the animal cell. Overall, animal metabolism produces energy in a opposite procedure to that of photosynthesis.
Creature metabolism produces energy in a reverse process to that of photosynthesis in plants.
Structure and Classification
I method of classifying carbohydrates is based on the number of carbon atoms per each molecule of a carbohydrate and on the number of molecules of sugar in the chemical compound. Based on the number of carbon atoms, a carbohydrate tin can be classified as triose (iii C), tetrose (four C), pentose (5 C), and hexose (6 C). The suffix "ose" at the end of a biochemical proper noun flags the molecule as a "saccharide." Amongst these, pentoses (eastward.chiliad., ribose in ribonucleic acid (RNA)) and hexoses (e.yard., glucose, or claret sugar) are the most common sugars in animal tissues. Based on the number of molecules of carbohydrate in the chemical compound, carbohydrates can be classified as (1) monosaccharide, one unit of sugar; (2) disaccharide, 2 monosaccharides; (three) oligosaccharide, three to fifteen monosaccharides; and (4) polysaccharides, large polymers of elementary sugars.
A. Monosaccharides are oftentimes referred to as unproblematic sugars (e.g., glucose) and cannot exist hydrolyzed into simpler compounds.
Monosaccharides can be subdivided based on the number of carbon (C) atoms. The following listing shows the prefixes for numbers of carbons in a saccharide.
- Triose (3 C)
- Tetrose (iv C)
- Pentose (five C; eastward.thousand., Xylose and Ribose)
- Hexose (6 C; e.one thousand., glucose, fructose, galactose, and mannose)
Monosaccharides are the simplest forms of carbohydrate.
Most monosaccharides in animate being tissues are of 5 C and 6 C sugars. Simple sugars are too subdivided into aldose, a sugar that contains an aldehyde structure, or ketose, a saccharide that contains a ketone group. Both glucose and fructose take the same molecular formula C6H12O6 and are hexoses (6 C). Only glucose is an aldose (also called aldohexose) and fructose is a ketose, or a ketohexose.
The iii hexoses that are nutritionally and metabolically important are glucose, fructose, and galactose (meet Figure 3.1).
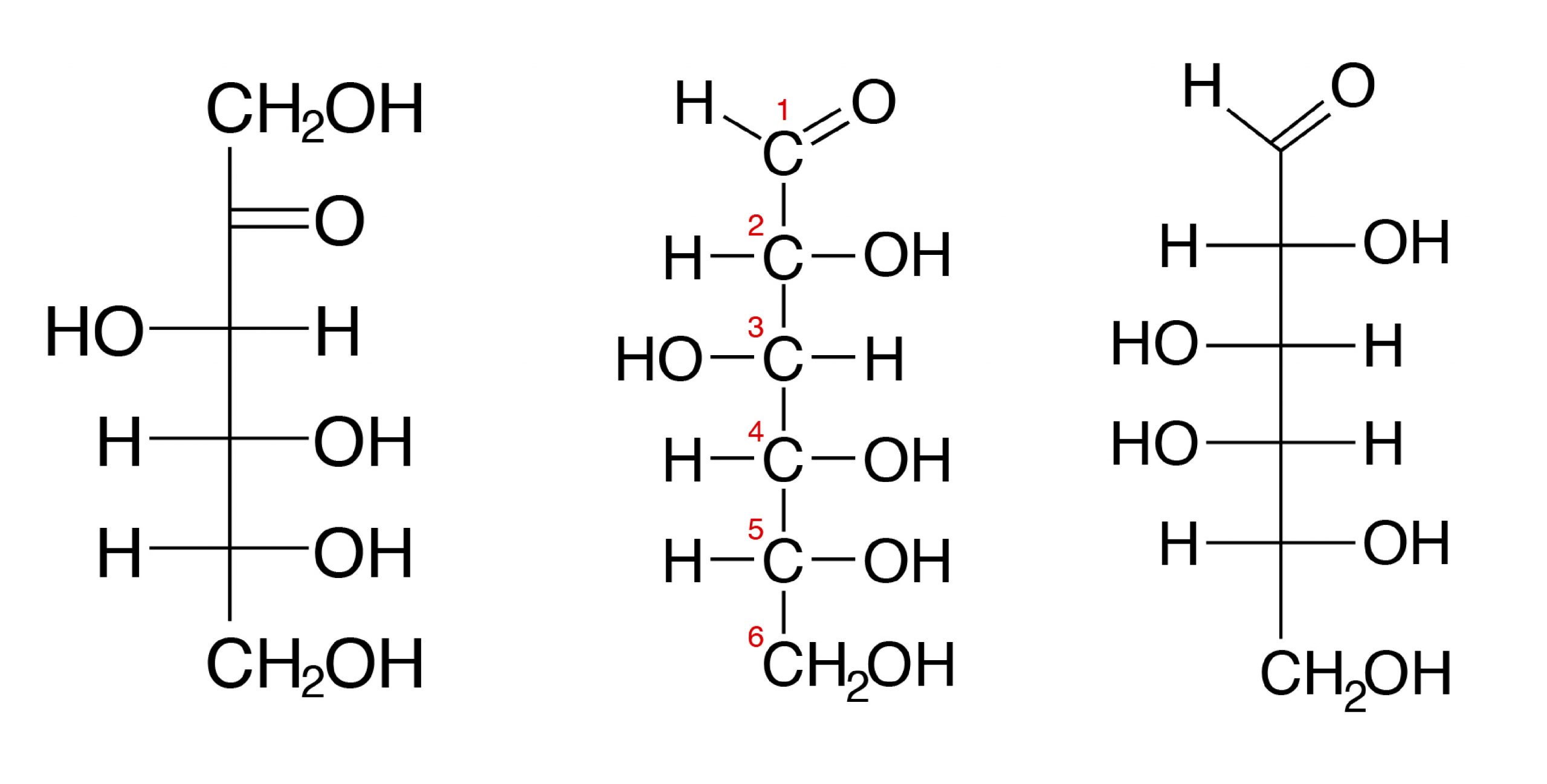
Most nutritionally important sugars are pentoses or hexoses.
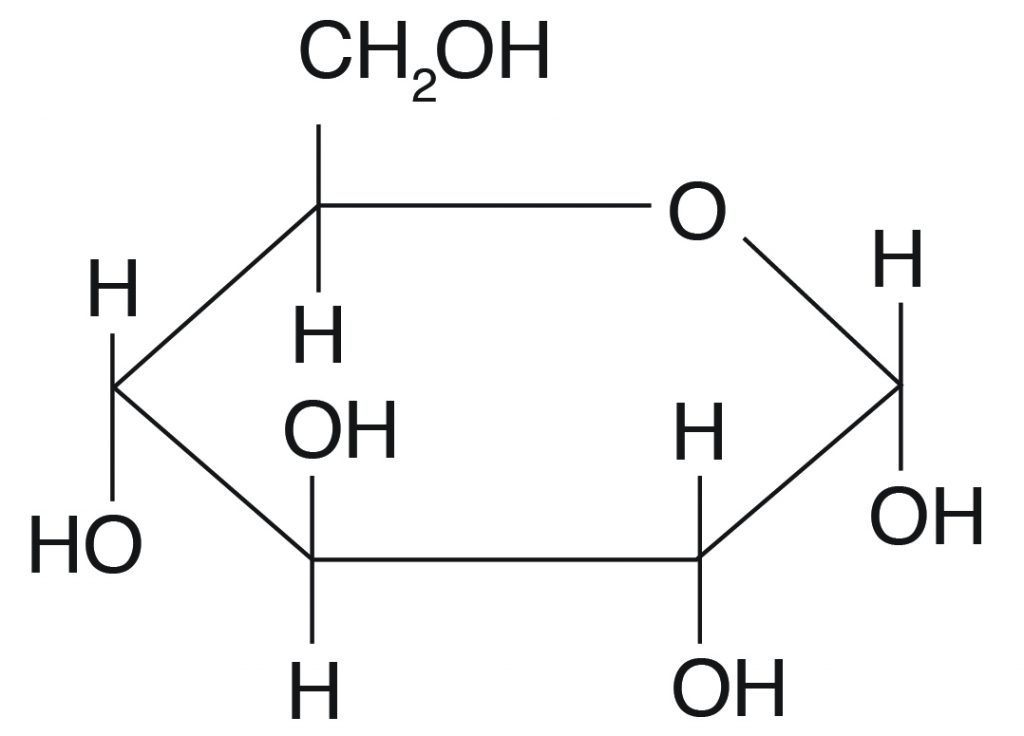
The chemical construction of glucose can be represented as a direct concatenation form (Figure 3.ane) and in cyclic form (also shown in Figure 3.1). In a biological system, glucose exists primarily as a cyclic form and very rarely in a straight form (in aqueous solution). Glucose is the form of carbohydrates institute in circulating blood (blood sugar) and is the primary saccharide used past the body for energy production. Fructose, or "fruit sugar," is found in ripened fruits and honey and is besides formed by digestion of disaccharide sucrose. Galactose is found along with disaccharide lactose in mammalian milk and is released during digestion.
Glucose can exist as α and β isomers and has immense animal nutritional implications. These two isomers differ in their orientation of OH on C #i (shown in red in Effigy 3.ii).
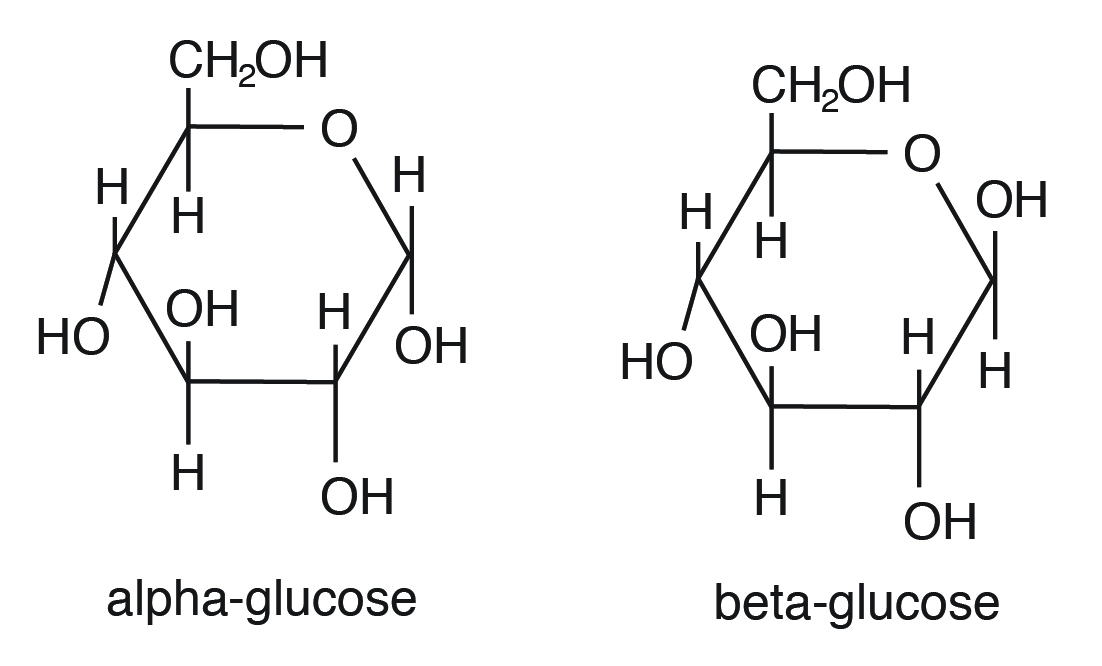
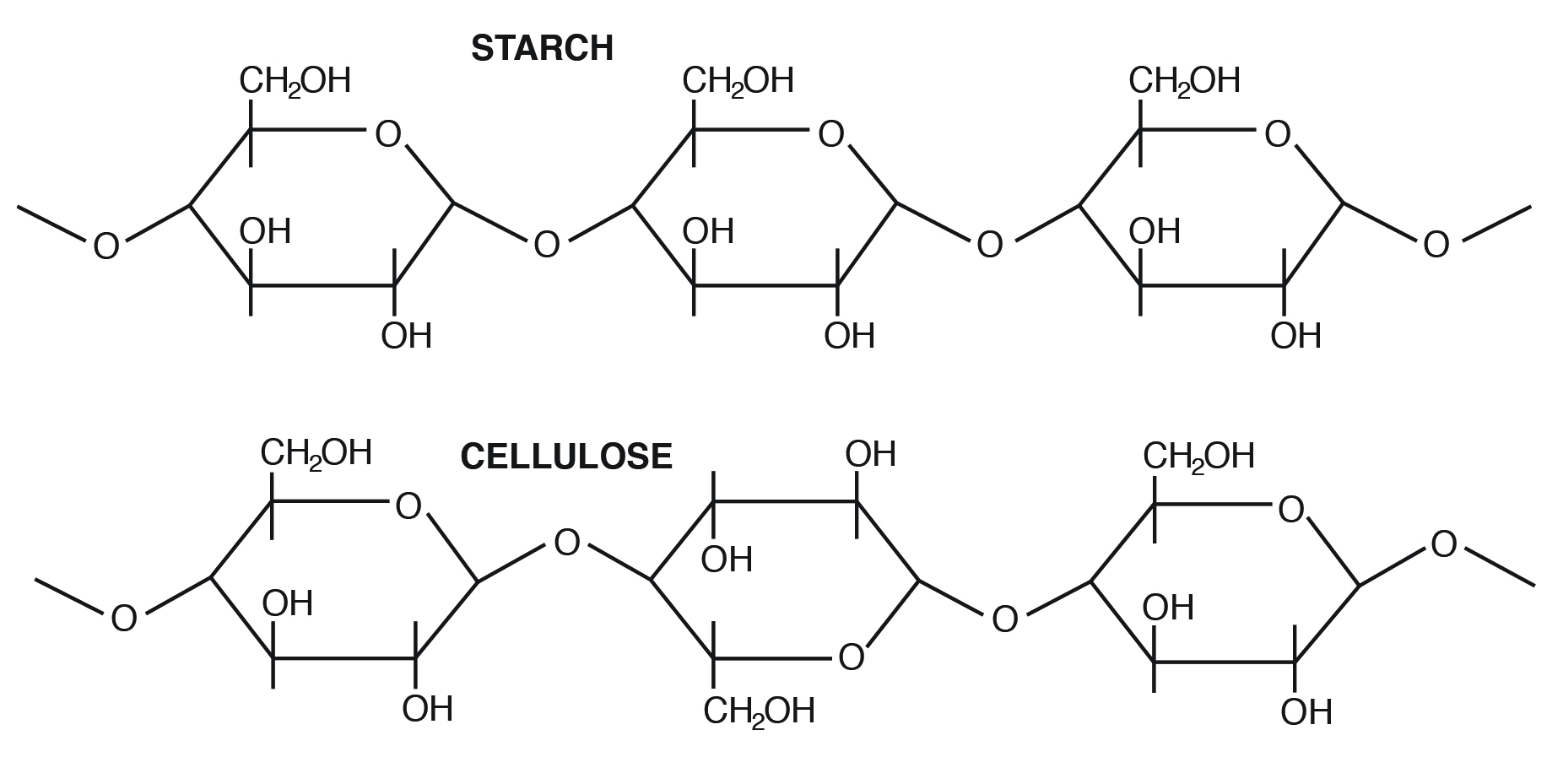
For instance, starch contains α-D-Glucose, while cellulose has rigid polymers with β-D-Glucose. Nutritionally important sugars are of the D-form (not the L-course). D and Fifty refer to stereo-orientation at disproportionate carbon position 5 in a hexose or carbon position 4 in a pentose.
Nutritional important sugars are of the D-form.
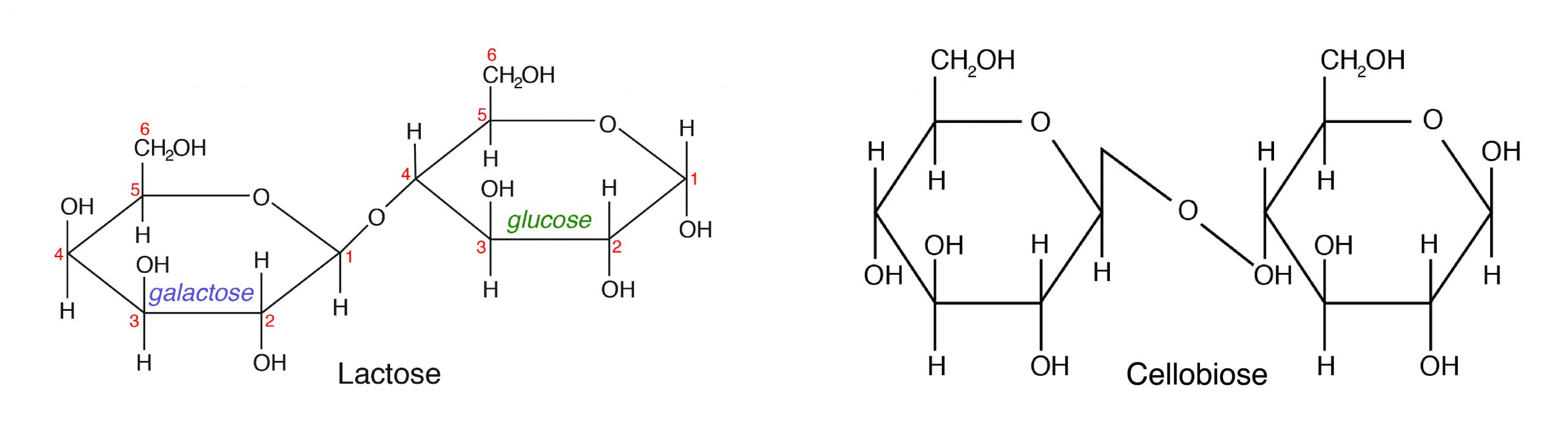
B. Disaccharides are made up of two monosaccharides bonded together by a glycosidic (covalent) bond. The following are some of the common disaccharides:
- Sucrose-glucose + fructose (e.k., tabular array saccharide)
- Lactose-glucose + galactose (milk sugar)
- Maltose-α-D-Glucose + β-D-Glucose (malt carbohydrate)
- Cellobiose-β-D-Glucose + β-D-Glucose (cellulose)
Amidst the dissimilar disaccharides, lactose (milk sugar) is the only sugar of animal origin. Notwithstanding, cellobiose as a component of cellulose is important in animal nutrition. Monogastric animals cannot digest cellulose because they do non produce the cellulase enzyme that tin can split β-D-Glucose.
C. Oligosaccharide are made by bonding together 3 or more (3 to fifteen) monosaccharides bonded together.
- Raffinose (glucose + fructose + galactose; 3 sugars)
- Stachyose (glucose + fructose + 2 galactose; iv sugars)
In animal diets, oligosaccharides are ordinarily found in beans and legumes. Some oligosaccharides are used equally substances to enhance the growth of good microbes (prebiotics). Recently, there has been an increased interest in the utilise of unlike oligosaccharides as feed additives to enhance hindgut health (east.g., fructooligosaccharides, mannan oligosaccharides).
D. Polysaccharides, equally their name implies, are fabricated past joining together large polymers of unproblematic sugars.
Polysaccharides are the nigh important carbohydrate in animal feed. Polysaccharides are equanimous of many single monosaccharide units linked together in long, complex chains. The functions of polysaccharides include energy storage in plant cells (e.1000., seed starch in cereal grains) and brute cells (due east.g., glycogen) or structural support (establish fiber). Components of cell wall structure are also chosen nonstarch polysaccharides, or resistant starch, in animal nutrition, as they cannot be digested by beast enzymes simply are fermented by hindgut and rumen microbes.
Polysaccharides can exist homopolysaccharides or heteropolysaccharides.
- a. Homopolysaccharide
- b. Heteropolysaccharide
a. Homopolysaccharide: Contains only one type of carbohydrate unit.
Examples of homopolysaccharides that are important in animal nutrition include starch (nonstructural form), glycogen (animal class), and cellulose (plant structural form).
- Starch: Principal carbohydrate form of carbohydrate in cereal grains (seed free energy storage). The bones unit is α-D-Glucose. Forms of starch in cereal grains include
- Amylose-α one,4 linkage-straight chain, nonbranching, helical construction
- Amylopectin-α i,iv linkage with blastoff 1,6 linkage at branch points
Amylose is the simplest of the polysaccharides, being comprised solely of glucose units joined in an alpha one,iv linkage (Figure 3.4). Amylose is h2o soluble and constitutes 15% to 30% of total starch in most plants.
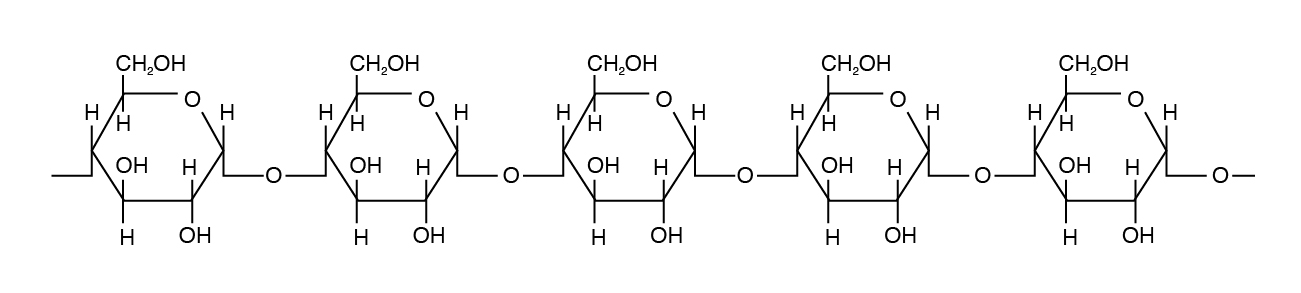
Amylopectin differs in how the glucose units are joined together. Alpha ane,iv linkages predominate, only a "branch" arises from an alpha 1,six linkage. Such branches make the structure of amylopectin more complex than that of amylose. Amylopectin is not water soluble and constitutes 70% to 85% of total starch in constitute cells.
Starch is the chief sugar source in the diet of monogastric animals.
Amylopectin is the major form of starch in institute cells.
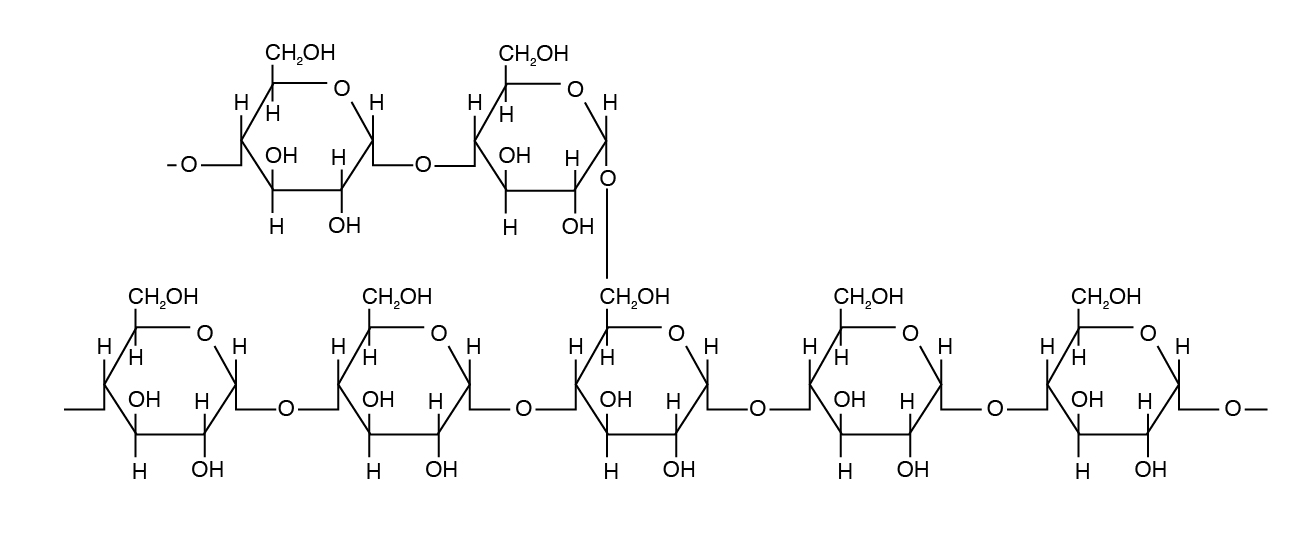
Glycogen is a form of starch found in beast tissue and is hence called animal starch. Glycogen is a polysaccharide that is physically related to amylopectin with basic blastoff-D-Glucose but has a mix of α 1,4 and α one,half-dozen bonds. Glycogen exists in a minor amount (< one%) in liver and muscle tissue.
Cellulose is the most arable sugar in nature. It provides structural integrity to establish jail cell walls. The basic unit is β one,4 linkage, direct chain, nonbranching (Figure 3.three). Cellulose is highly stable. No animal enzyme tin pause information technology; only microbial cellulase tin can degrade it. Ruminant animals such as cattle, however, take bacteria in their rumen that contain the enzyme cellulase. It breaks the beta one,4 links of the glucoses in cellulose to release the saccharide for energy.
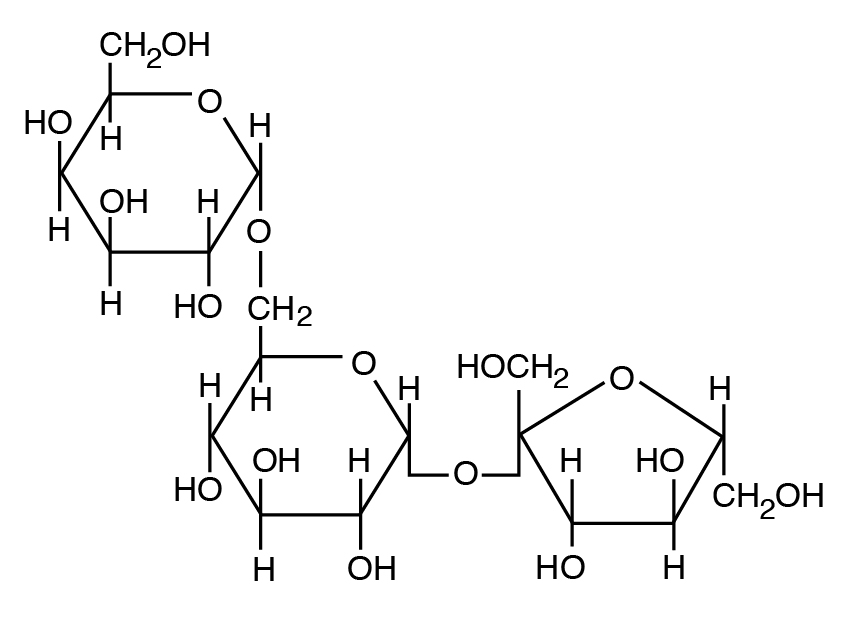
b: Heteropolysaccharide: A component of institute prison cell walls with a mix of 5 C and 6 C sugars (e.thousand., hemicellulose and pectin, a mixture of pentose and hexose units).
Fundamental Points
- Carbohydrates are "hydrates of carbon" and have the generic construction of C(north)H(2n)O(n).
- A single sugar unit is a monosaccharide. These can consist of 3-carbon moieties (triose), 4-carbon units (tetrose), 5-carbon moieties (pentose), and 6-carbon moieties (hexose).
- Most nutritionally important sugars are pentoses or hexoses.
- Further classification of sugars is a definition of either aldose (having an aldehyde group) or ketose (having a ketone grouping). Glucose, mannose, and galactose are aldoses, whereas fructose is a ketose.
- Nutritionally important sugars are of the D-form (not the L-form). D and L refer to stereo-orientation at asymmetric carbon position 5 in a hexose or carbon position 4 in a pentose.
- Sugars link together via a glycosidic bond to form di- (two monosaccharides) or oligo- (3 to 15 monosaccharides), and polysaccharides.
- The nature of glycosidic bonds influences the structural and chemic properties of the sugars and influences their ease of digestion. Sugars that bond via an alpha ane,four linkage may be digested by mammalian enzymes. Sugars that are linked via the beta 1,iv linkage are resistant to digestion.
- Nutritionally significant disaccharides are sucrose and lactose.
- Starch from plants serves as a major energy source in creature diets. Starch consists of two types of molecules: amylose (blastoff one,4 linked glucose) and amylopectin (blastoff 1,4 and blastoff i,6 linked glucose).
- Glycogen, a storage form of carbohydrates in the liver and muscles, is very like to starch also called animal starch.
- Plant polysaccharides also include cellulose and hemicellulose and pectin (nonstarch polysaccharides). Mammalian enzymes cannot degrade these polysaccharides to gratis sugars, only microbial enzymes can handle them.
Review Questions
- In what of import ways practice starch and cellulose differ?
- What are the disaccharides of nutritional significance?
- Nutritional important sugars are of the D-form or the L-class?
- The well-nigh important carbohydrate in nutrition
- Listing the two forms in which starch exist
- The forms of starch in the animal body is?
- A structural homopolysaccharide made of glucose is
- cellulose
- hemicellulose
- pectin
- raffinose
- Amidst these unlike sugars, the master source of energy for a broiler craven is
- fructose
- sucrose
- glycogen
- glucose
- Two molecules of saccharide are linked together by this bail
- peptic bond
- glycosidic bond
- diglyceride bail
- both a) and b)
- Amid the two forms of starch, this is the major component of cereal grains
- amylose
- amylopectin
- cellulose
- glycogen
Source: https://open.oregonstate.education/animalnutrition/chapter/chapter-3/
Posted by: hookcounces.blogspot.com

0 Response to "What Is The Stored Carbohydrate In Animals"
Post a Comment